CcRgl11A(Rhamnogalacturonan lyase)
CcRgl11A
En-Rgl0189
(EC. 4.2.2.23) Rhamnogalacturonan lyase
CAZy Family: PL11
PROPERTIES
1. ELECTROPHORETIC PURITY
-Single band on SDS-gel electrophoresis (MW ~72 kDa)
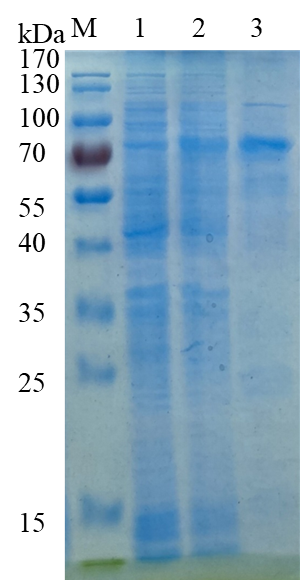
Fig 1. Electrophoresis analysis of CcRgl11A. M, molecular weight marker (PageRuler Prestained Protein Ladder, Thermo Scientific); lane 1, culture lysate before IPTG induction; lane 2, culture lysate after IPTG induction; lane 3, CcRgl11A purified from Ni sepharose fast flow column.
2. SPECIFIC ACTIVITY
0.9 U/mg protein (on RG-I-AT from Adenophora tetraphylla(Thunb.)Fisch.) at pH8.0 and 37°C.
One unit of RGL activity was defined as the amount of enzyme required to generate 1 mmol of 4,5-unsaturated galacturonic acid in 1 min.
3. RELATIVE RATES OF HYDROLYSIS OF SUBSTRATES
The activity of Rgl11Y was tested on four different substrates (polygalacturonic acid, pectin from citrus, PPG, and rhamnogalacturonan). In a standard assay, the specific activity of Rgl11Y was found to be of 28 and 3.6 IU/mg, respectively, using PPG and rhamnogalacturonan as substrates. No activity against polygalacturonic acid and pectin from citrus could be detected, indicating that Rgl11Y is not an HG-degrading enzyme. Rgl11Y cleaves specifically the backbone of RGI in pectin.
4. PHYSICOCHEMICAL PROPERTIES
pH Optima: 8.5
Temperature Optima: 37°C
5. STORAGE CONDITIONS
The enzyme should be stored at -20°C. For assay, this enzyme should be diluted in phosphate buffer (50 mM) pH 8.0. Swirl to mix the enzyme immediately prior to use.
6. REFERENCE
[1] Pagès S, Valette O, Abdou L, et al. A rhamnogalacturonan lyase in the Clostridium cellulolyticum cellulosome[J]. Journal of bacteriology, 2003, 185(16): 4727-4733.


