BaAra43A (exo-α-arabinofuranosidase,AXH-d3)
BaAra43A (AXH-d3)
Exo-Gal0153
(EC. 3.2.1.55) exo-α-L-arabinofuranosidase
CAZy Family: GH43
PROPERTIES
1. ELECTROPHORETIC PURITY
-Single band on SDS-gel electrophoresis (MW ~59 kDa)
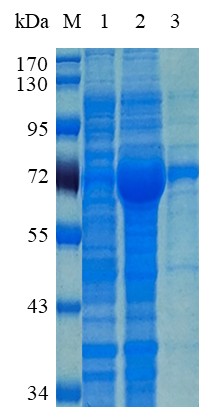
Figure 1. Electrophoresis analysis of BaAra43A. M, molecular weight marker (PageRuler Prestained Protein Ladder, Thermo Scientific); lane 1, culture lysate before IPTG induction; lane 2, culture lysate after IPTG induction; lane 3, BaAra43A purified from Ni sepharose fastflow column.
2. SPECIFIC ACTIVITY
No activity on pNPαAra. Only C3-linked arabinose residues from double-substituted xylose residues.
3. RELATIVE RATES OF HYDROLYSIS OF SUBSTRATES
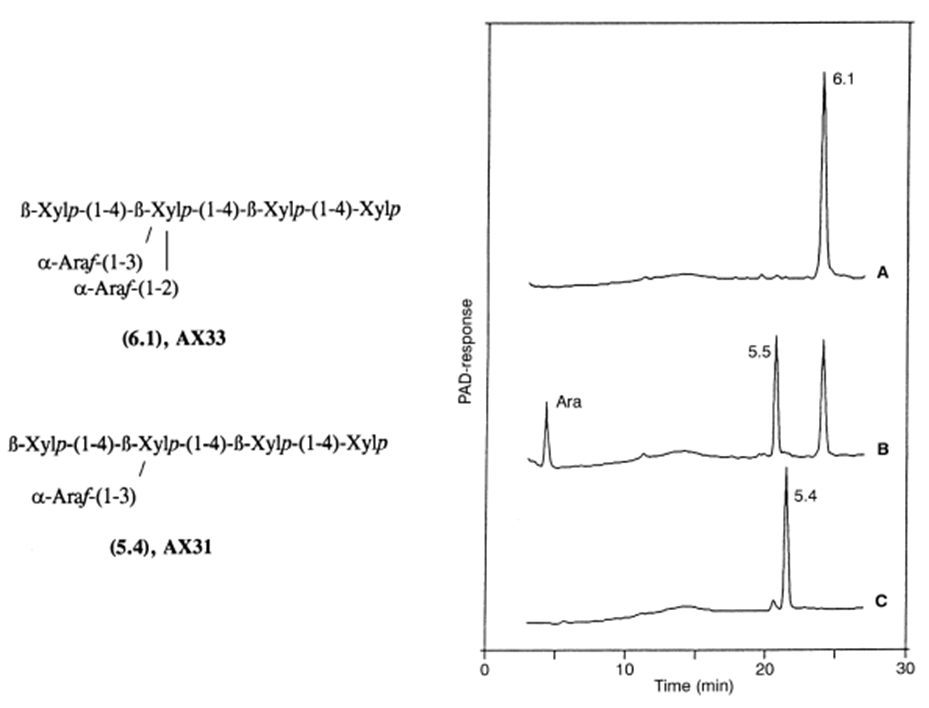
Figure 2. A–C High-performance anion-exchange analysis of the reaction products obtained after incubation of the oligosaccharide 6.1 with arabinofuranohydrolase. A Untreated oligosaccharide 6.1; B oligosaccharide 6.1 treated with arabinofuranohydrolase; C standard oligosaccharide 5.4.
4. PHYSICOCHEMICAL PROPERTIES
pH Optima: suggested use 6.0
Temperature Optima: suggested use 30°C
5. STORAGE CONDITIONS
The enzyme should be stored at -20°C. For assay, this enzyme should be diluted in phosphate buffer (20 mM) pH 7.0. Swirl to mix the enzyme immediately prior to use.
6. REFERENCES
[1] Van Laere KM, Beldman G, Voragen AG. A new arabinofuranohydrolase from Bifidobacterium adolescentis able to remove arabinosyl residues from double-substituted xylose units in arabinoxylan. Appl Microbiol Biotechnol. 1997 Mar;47(3):231-5.


