EoFuc29A(exo-α-Fucosidase)
EoFuc29A
Ex-Fuc0232
(EC 3.2.1.51) exo-α-Fucosidase
CAZy Family: GH29
PROPERTIES
1.ELECTROPHORETIC PURITY
-Single band on SDS-gel electrophoresis (MW ~49kDa)
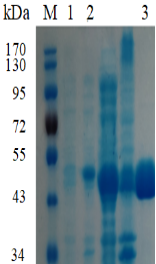
Figure.1 Electrophoresis analysis of EoFuc29A. M, molecular weight marker (PageRuler Prestained Protein Ladder, Thermo Scientific); lane 1, culture lysate before IPTG induction; lane 2, culture lysate after IPTG induction; lane 3, EoFuc29A purified from Ni sepharose fastflow column.
2.SPECIFIC ACTIVITY
0.11 U/mg protein (on pNP-α-fuc) at pH 8.0 and 37°C
One Unit of pNP-α-fuc activity is defined as the amount of enzyme required to release 1 μmol of p-nitrophenyl per hour from pNP-α-fuc (5 mM) in Tris-HCl buffer (20 mM) pH 8.0.
3.RELATIVE RATES OF HYDROLYSIS OF SUBSTRATES
Table 1. Relative activity ofEoFuc29Aon different substratesa.
Substrateb |
Relative activity (%)c |
pNPαGlc |
_ |
pNPβGlc |
_ |
pNPαGal |
474±5.8 |
pNPβGal |
_ |
pNPαMan |
_ |
pNPβMan |
_ |
pNPαXyl |
_ |
pNPβXyl |
_ |
pNPαAraf |
_ |
pNPαArap |
_ |
pNPαRha |
_ |
100±0.0 |
aReactions were performed with 5 mM substrate, pH 8.0, at 37°C for 30 min.
bAbsorption caused by released p-nitrophenol was measured at 405 nm. The relative activity on pNPαFuc was taken as 100%.
cThe data are reported as means±standard errors from the mean for three independent experiments.
4.PHYSICOCHEMICAL PROPERTIES
pH Optima:7.0
pH Stability:50°C
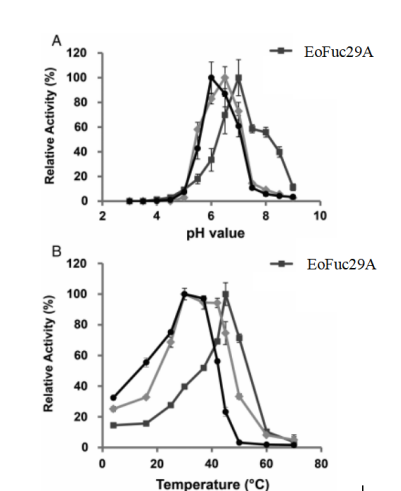
Figure3.Dependency of fucosidase activities on pH (A), temperature (B).
5.STORAGE CONDITIONS
The enzyme should be stored at-20°C. For assay, this enzyme should be diluted insodium Trisbuffer (20 mM) pH8.0. Swirl to mix the enzyme immediately prior to use.
6. REFERENCES
[1]Liu S, Kulinich A, Cai Z P, et al. The fucosidase-pool of Emticicia oligotrophica: biochemical characterization and transfucosylation potential[J]. Glycobiology, 2016, 26(8): 871-879.


