EmFuc29A(exo-α-Fucosidase)
EmFuc29A
Ex-Fuc0260
(EC 3.2.1.51) exo-α-Fucosidase
CAZy Family: GH29
PROPERTIES
1.ELECTROPHORETIC PURITY
-Single band on SDS-gel electrophoresis (MW ~52kDa)
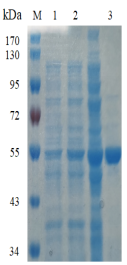
Fig.1 Electrophoresis analysis of EmFuc29A. M, molecular weight marker (PageRuler Prestained Protein Ladder, Thermo Scientific); lane 1, culture lysate before IPTG induction; lane 2, culture lysate after IPTG induction; lane 3, EmFuc29A purified from Ni sepharose fastflow column.
2.SPECIFIC ACTIVITY
0.10 U/mg protein (on pNP-α-fuc) at pH 7.0 and 37°C
One Unit of pNP-α-fuc activity is defined as the amount of enzyme required to release 1 μmol of p-nitrophenyl per hour from pNP-α-fuc (5 mM) in sodium phosphate buffer (20 mM) pH 7.0.
3.RELATIVE RATES OF HYDROLYSIS OF SUBSTRATES
Table 1. Relative activity of BfFuc29B on different substratesa.
Substrateb |
Relative activity (%)c |
pNPαGlc |
_ |
pNPβGlc |
_ |
pNPαGal |
_ |
pNPβGal |
_ |
pNPαMan |
_ |
pNPβMan |
_ |
pNPαXyl |
_ |
pNPβXyl |
_ |
pNPαAraf |
_ |
pNPαArap |
_ |
pNPαRha |
_ |
100±0.0 |
aReactions were performed with 5 mM substrate, pH 7.0, at 37°C for 30 min.
bAbsorption caused by released p-nitrophenol was measured at 405 nm. The relative activity on pNPαFuc was taken as 100%.
cThe data are reported as means±standard errors from the mean for three independent experiments.
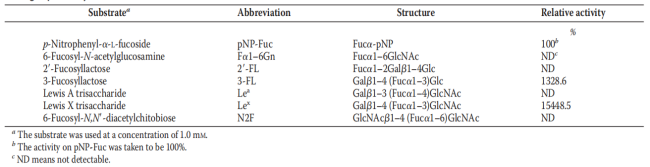
Table 2 Linkage specificity of EmFuc29A
4.PHYSICOCHEMICAL PROPERTIES
pH Optima:4.5
pH Stability:4-6
Temperature Optima:55°C
Temperature Stability:<45°C
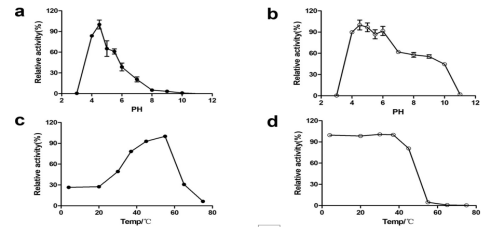
Figure 2. Enzymatic characterization of purified recombinant EmFuc29A. The enzymatic activity and stability of EmFuc29A at various pH values (a and b) and temperatures (c and d) are shown.
5.STORAGE CONDITIONS
The enzyme should be stored at -20°C. For assay, this enzyme should be diluted in sodium phosphate buffer (20 mM) pH 7.0. Swirl to mix the enzyme immediately prior to use.
6. REFERENCES
[1]Li, Tiansheng, Mengjie, et al. Identification and characterization of a core fucosidase from the bacterium Elizabethkingia meningoseptica[J]. Journal of Biological Chemistry, 2018, 293(4): 1243-1258.


