SvRha90A(endo-α-1,3-Rhamnosidase)
SvRha90A
Endo-Rha0198
(EC.3.2.1) endo-α-1,3-Rhamnosidase
CAZy Family: GH90
PROPERTIES
1. ELECTROPHORETIC PURITY
-Single band on SDS-gel electrophoresis (MW ~74 kDa)
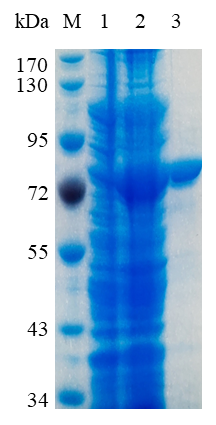
Figure 1. Electrophoresis analysis of SvRha90A. M, molecular weight marker (PageRuler Prestained Protein Ladder, Thermo Scientific); lane 1, culture lysate before IPTG induction; lane 2, culture lysate after IPTG induction; lane 3, SvRha90A purified from Ni sepharose fastflow column.
2. SPECIFIC ACTIVITY
2.3 U/mg protein (on Dodecasaccharide) at pH7.0 and 37°C.
One Unit of endo-rhamnosidase activity is defined as the amount of enzyme required to release 1 μmol of reducing sugar per minute from Dodecasaccharide (5 mM) in phosphate buffer (60 mM) pH 7.0.
3. RELATIVE RATES OF HYDROLYSIS OF SUBSTRATES
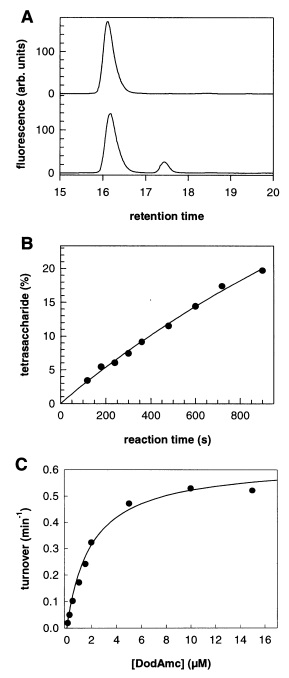
Figure 2. SvRha90A endorhamnosidase activity measured with a defined substrate. (A) Reversed-phase HPLC elution profiles of purified DodAmc (15 µM, upper trace) and of a sample that was incubated with purified SvRha90A (170 nM subunits) for 15 min at 10 °C. (B) Time course of DodAmc hydrolysis by SvRha90A (0.5 µM subunits) at 10 °C. The substrate concentration was 15 µM; the solid line represents a simulation assuming rapid equilibration with substrate and product, and using turnover number, substrate KM, and product dissociation constants determined in the present study. The simulation was done with KINSIM. (C) Substrate concentration dependence of DodAmc turnover at 10 °C. The solid line represents a nonlinear fit to the Michaelis-Menten equation, resulting in KM = 2.3 µM and kcat = 0.01 s-1. The SvRha90A subunit concentration was 170 nM.
4. PHYSICOCHEMICAL PROPERTIES
pH Optima: suggested use 7.0
Temperature Optima: suggested use 37°C
5. STORAGE CONDITIONS
The enzyme should be stored at -20°C. For assay, this enzyme should be diluted in phosphate buffer (20 mM) pH 7.0. Swirl to mix the enzyme immediately prior to use.
6. REFERENCES
[1] Andres D, Hanke C, Baxa U, Seul A, Barbirz S, Seckler R. Tailspike interactions with lipopolysaccharide effect DNA ejection from phage P22 particles in vitro. J Biol Chem. 2010 Nov 19;285(47):36768-75.


