MeGlu3A (exo-β-Glucosidase)
MeGlu3A
Ex-Glu0018
(EC.3.2.1.21) exo-β-Glucosidase
CAZy Family: GH3
PROPERTIES
1.ELECTROPHORETIC PURITY
-Single band on SDS-gel electrophoresis (MW ~80kDa)
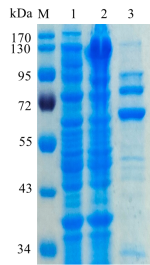
Figure 1. Electrophoresis analysis of MeGlu3A. M, molecular weight marker (PageRuler Prestained Protein Ladder, Thermo Scientific); lane 1, culture lysate before IPTG induction; lane 2, culture lysate after IPTG induction; lane 3, MeGlu3A purified from Ni sepharose fastflow column.
2.SPECIFIC ACTIVITY
29.4 U/mg protein (on pNP-β-glu) at pH 7.0 and 40oC
One Unit of pNP-β-glu activity is defined as the amount of enzyme required to release one μmole of glucose per minute from pNP-β-glu (5 mM) inphosphate buffer (10 mM) pH 7.0.
3.RELATIVE RATES OF HYDROLYSIS OF SUBSTRATES
Table 1.Kinetic parameters for MeGlu3A on different substrates.
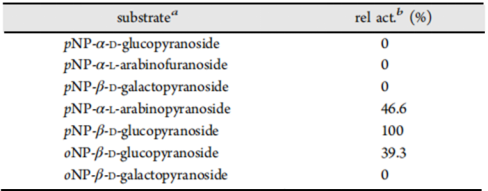
a Final concentration of each 10.0 mM.
b The relative enzyme activity against of pNP-β-D-glucopyranoside was assumed to be 100%.

Figure 2. TLC analysis of the time-course transformation of ginsenoside Rb1 by MeGlu3A.

Figure 3. Transformation of ginsenosides Rb1, Rc, Rd, and F2 to compound Mc and compound K by MeGlu3A. Metabolites were analyzed by TLC.
4.PHYSICOCHEMICAL PROPERTIES
pH Optima:7.0
pH Stability:4.0-6.0
Temperature Optima:40oC
Temperature Stability:<20oC
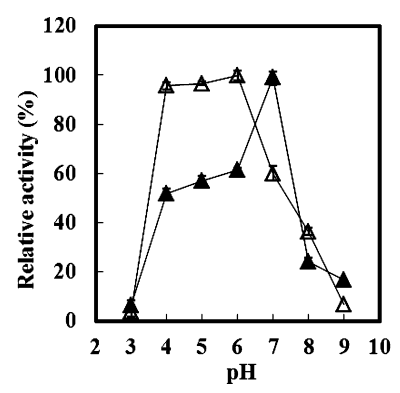
Figure4. Effect of pH on the stability and the activity of recombinant MeGlu3A determined using pNP-β-D-glucopyranoside as a substrate. The following buffers (20 mM) were tested: glycine/HCl buffer (pH 3.0), citric acid/sodium citrate buffer (pH 4.0−5.0), sodium phosphate buffer (pH 6.0−7.0), and Tris/HCl buffer (pH 8.0−9.0). ▲, enzyme activity. Maximum activity observed at pH 7.0 was taken as 100% = 29.4 U (mg protein)−1. Δ, stability. Enzyme activity was assayed under standard conditions (pH 7.0, 40 °C) after incubation at 40 °C for 4 h at different pH levels. The original activity before incubation was taken as 100%.
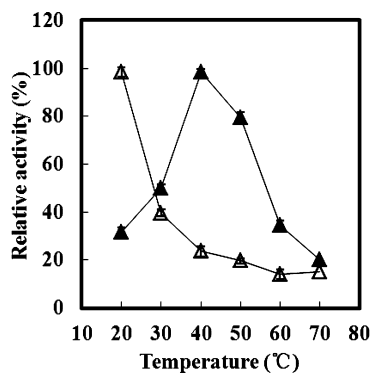
Figure5. Effect of temperature on the stability and activity of recombinant MeGlu3A determined using pNP-β-D-glucopyranoside as a substrate. ▲, the thermodependence of enzyme activity was assayed in 20 mM sodium phosphate buffer (pH 7.0) at various temperatures ranging from 20 to 70 °C. Maximum activity observed at 40 °C was taken as 100% = 29.4 U (mg protein)−1. Δ, thermostability was tested by incubating aliquots of the enzyme in 20 mM sodium phosphate buffer (pH 7.0) for 4 h at different temperatures.
5.STORAGE CONDITIONS
The enzyme should be stored at -20°C. For assay, this enzyme should be diluted in phosphate buffer (10 mM) pH 7.0. Swirl to mix the enzyme immediately prior to use.
6. REFERENCES
[1] Lin-Hu Quan, Jin-Woo Min, Yan Jin, et al.Enzymatic Biotransformation of Ginsenoside Rb1 to Compound K by Recombinant β-Glucosidase from Microbacterium esteraromaticum. Journal of Agricultural and Food Chemistry, 2012, 60: 3376-3378.


