PmGlu3C(exo-β-Glucosidase)
PmGlu3C
Ex-Glu0326
(EC.3.2.1.21) exo-β-Glucosidase
CAZy Family: GH3
PROPERTIES
1. ELECTROPHORETIC PURITY
-Single band on SDS-gel electrophoresis (MW ~105 kDa)
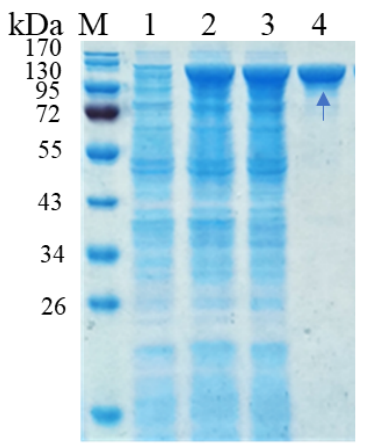
Figure 1. Electrophoresis analysis of PmGlu3C. M, molecular weight marker (PageRuler Prestained Protein Ladder, Thermo Scientific); lane 1, culture lysate before IPTG induction; lane 2, culture lysate after IPTG induction; lane 3, PmGlu3C purified from Ni sepharose fastflow column.
2. SPECIFIC ACTIVITY
3.11 U/mg protein (on pNP-β-glu) at pH 6.0 and 40°C
One Unit of pNP-β-glu activity is defined as the amount of enzyme required to release one μg of glucose per minute from pNP-β-glu (5 mM) in phosphate buffer (50 mM) pH 6.0.
3. RELATIVE RATES OF HYDROLYSIS OF SUBSTRATES
Table 1. Relative activity of PmGlu3C on different substrates.
Substrate |
Relative activity (±SD) |
pNPβGlc |
100±0.0 |
pNPβGal |
_ |
pNPβMan |
_ |
pNPβXyl |
_ |
pNPαGlc |
_ |
pNPαGal |
_ |
pNPαMan |
_ |
pNPαAraf |
_ |
pNPαArap |
_ |
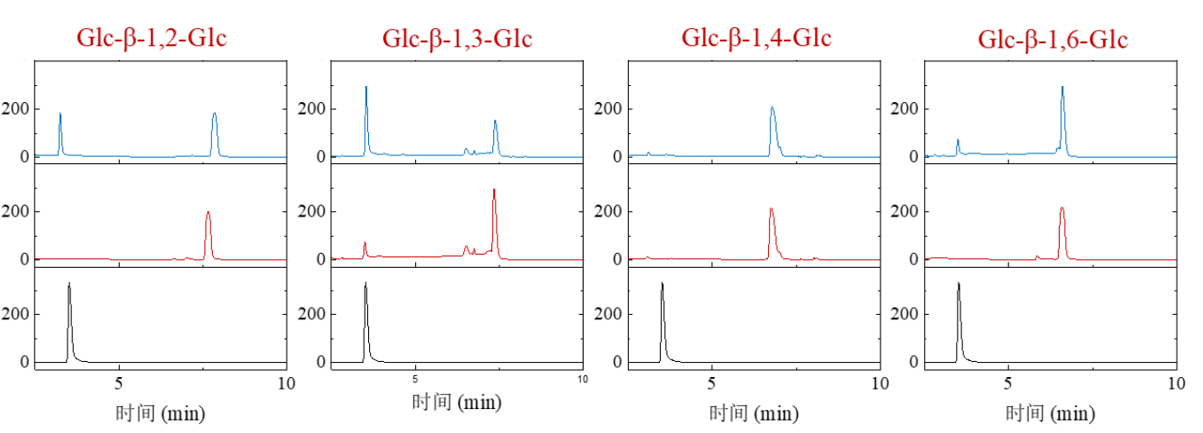
Figure 2. Sophorose (Glc-β-1,2-Glc), Laminaribiose (Glc-β-1,3-Glc), Cellobiose (Glc-β-1,4-Glc) and Gentiobiose (Glc-β-1,6-Glc) were selected as substrates to detect substrate selectivity of PmGlc3C. PmGlc3C can partially hydrolyze sophorose and Laminaribiose, can weakly hydrolyze ginsenoside, indicating that PmGlc3C is an exo-β-1,2/1,3/1,6-glucosidase, and it shows stronger activity in hydrolyzing β-1,2/1,3-glycosidic bonds and weaker activity in hydrolyzing β-1,6-glycosidic bonds.
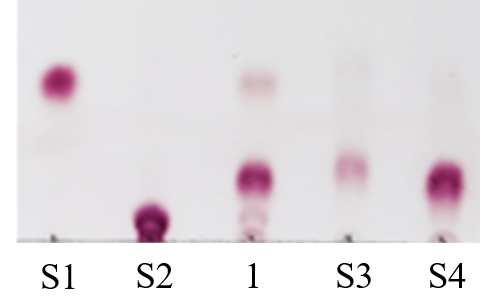
Figure. 3. Analysis of ginsenosidase activity of PmGlc3A by TLC. S1, standards of F2; S2, standards of Rb1; S3, standards of Rd; S4, standards of GYP XVII;S3, standards of GYP XVII;1, transformed products of Rb1. PmGlc3C can hydrolyze Rb1 to GYP XVII and trace F2. Hydrolysis of Rb1 to GYP XVII is the hydrolysis of glucose linked by β-1,2-glycosidic bond at C-3 position, and hydrolysis of Rb1 to F2 is the hydrolysis of glucose linked by β-1,2-glycosidic bond at C-3 position and glucose linked by β-1,6-glycosidic bond at C-20 position. The results indicate that PmGlc3C is an exo-β-1,2/1,6-glucosidase, and its ability to hydrolyze β-1,2-glycosidic bond is strong, while its ability to hydrolyze β-1,6-glycosidic bond is weak. Consistent with glucosamine hydrolysis results.
4. PHYSICOCHEMICAL PROPERTIES
pH Optima: 6.0
pH Stability: 5.0-9.0
Temperature Optima: 40 °C
Temperature Stability: 30-40 °C
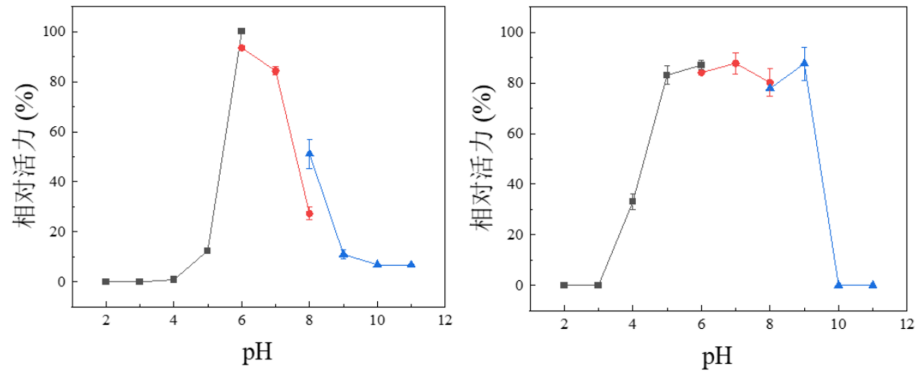
Figure 4. Effect of pH on activity (a) and stability (b) of PmGlu3C using pNP-Glu as substrate. The optimal pH (a) was determined at different pH from 2 to 11. The maximum activity obtained was defined as 100% activity. Thermal stability was determined by incubating the enzyme for 24 h at different pH. The activity of the enzyme before incubation was defined as 100%. Results are presented as means ± standard deviations(n = 3).
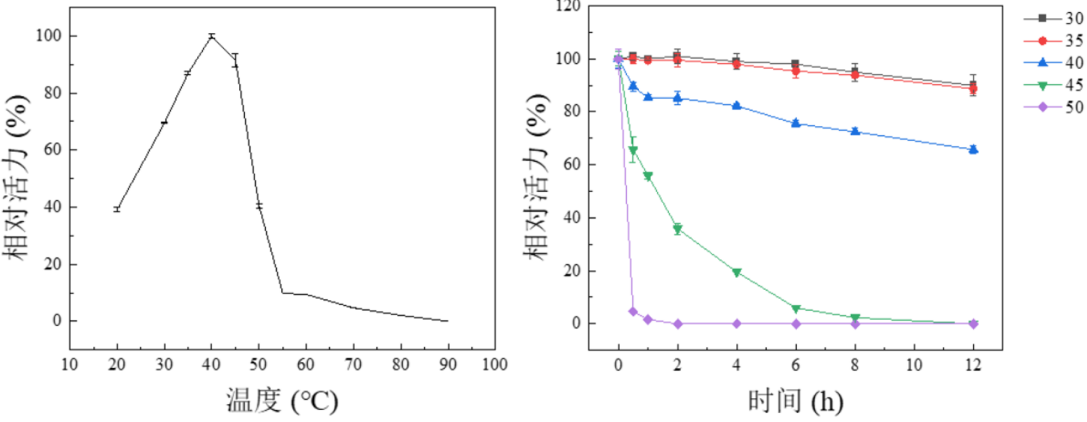
Figure 5. Effect of temperature on activity (a) and stability (b) of PmGlu3C using pNP-Glu as substrate. The optimal temperature (a) was determined at different temperatures from 20 to 90℃. The maximum activity obtained was defined as 100% activity. Thermal stability was determined by incubating the enzyme for 12 h at different temperatures. The activity of the enzyme before incubation was defined as 100%. Results are presented as means ± standard deviations(n = 3).
5. STORAGE CONDITIONS
The enzyme should be stored at -20°C. For assay, this enzyme should be diluted in phosphate buffer (20 mM) pH 6.0. Swirl to mix the enzyme immediately prior to use.
6. REFERENCES
[1]张国晶. 浸麻类芽孢杆菌中葡甘露聚糖降解酶重组表达及功能研究[D].东北师范大学,2022.
[2]孟嘉仪, 张国晶, 原野. 浸麻类芽孢杆菌葡甘露聚糖降解酶的异源表达及功能研究.微生物学报,2023,63(08):3129-3143.


