PcGal53A (endo-β-1,4-Galactanase)
PcGal53A
Endo-Gal0074
(EC.3.2.1.89)endo-β-1,4-Galactosidase
CAZy Family: GH53
PROPERTIES
1.ELECTROPHORETIC PURITY
-Single band on SDS-gel electrophoresis (MW ~38kDa)
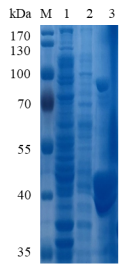
Figure 1. Electrophoresis analysis of PcGal53A. M, molecular weight marker (PageRuler Prestained Protein Ladder, Thermo Scientific); lane 1, culture lysate of E. coli BL21-pMAL-c2X-PcGal53A before IPTG induction; lane 2, culture lysate of E. coli BL21-pMAL-c2X-PcGal53A after IPTG induction; lane 3, PcGal53A purified from Ni sepharose fastflow column.
2.SPECIFIC ACTIVITY
7.6U/mg protein (on lupin galactan) at pH5.0 and 37oC.
One Unit ofgalactosidaseactivity is defined as the amount of enzyme required to release one μmole ofreducing sugarper minute from lupin galactan (0.1%)in Na-acetate buffer (20 mM) pH 5.0.
3.RELATIVE RATES OF HYDROLYSIS OF SUBSTRATES
Table 1 Enzyme activities ofrPcGal53Aand rPcGALX35C towards various plant polysaccharides
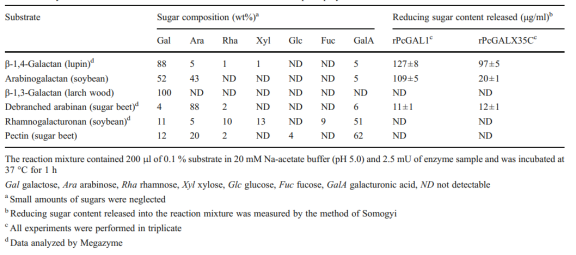
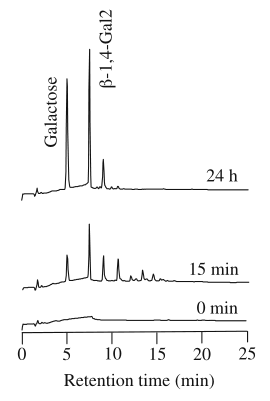
Figure 2. HPAEC chromatograms of degradation products of lupin galactan following incubation with PcGal53A for the indicated times.
Table2. Enzyme activities of PcGal53A towardsvarious oligosaccharides
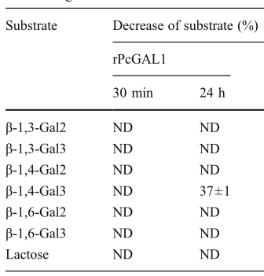
Enzyme activity was tested by incubating 100 μl of 300 μM substratein 20 mM Na-acetate buffer (pH 5.0) with the enzyme (12 mU forPcGal53A and 0.2 mU for rPcGALX35C) at 37 °C for the indicatedtimes. The reaction mixtures were analyzed with HPAEC under theconditions described in the text. The experiments were performed intriplicate.
ND not detectable
Table3. Degradation of polysaccharides in combination with PcGal53A, rPcGALX35C, α-L-arabinofuranosidase (rAFS1), and endo-α-1,5-arabinanase (rAbnc)
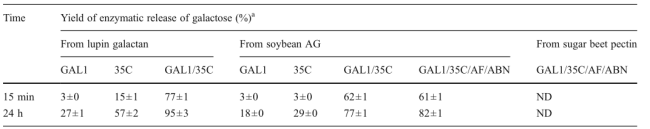
The experimental conditions are described in the text. The experiments were performed in triplicateND not detectablea Yields of release of galactose (percent) were calculated taking the amount of the total galactose in the substrate as 100 %. GAL1: PcGal53A, 35C:rPcGALX35C, AF: rAFS1, ABN: rAbnc
4.PHYSICOCHEMICAL PROPERTIES
pH Optima: 5.0
pH Stability: 3.0-9.0
Temperature Optima: 40°C
5.STORAGE CONDITIONS
The enzyme should be stored at -20 °C. For assay, this enzyme should be diluted in acetate buffer (20 mM) pH 5.0. Swirl to mix the enzyme immediately prior to use.
6. REFERENCES
[1] Sakamoto T, Nishimura Y, Makino Y, et al. Biochemical characterization of a GH53 endo-β-1,4-galactanase and a GH35 exo-β-1,4-galactanase from Penicillium chrysogenum. Appl Microbiol Biotechnol. 2013 Apr;97(7):2895-906.


